Abstract
Background: FVIII replacement therapy is ineffective for severe haemophilia A (HA) patients who develop inhibitors to FVIII. Patients with intractable inhibitors currently require FVIII mimetics and/or bypassing agents to prevent bleeding. PEGylated liposomes (PEGLip) have been shown to protect FVIII from anti-FVIII antibodies in ex-vivo human studies and in combination with FVIII may present an option for the prophylactic treatment of inhibitor patients.
Aims: To (a) demonstrate that PEGLip-FVIII administered intravenously (IV) to severe HA patients with history of inhibitors to FVIII enhances their clotting activity, (b) compare the number of bleeding episodes before and after PEG-Lip treatment, and (c) demonstrate that PEGLip-FVIII is well tolerated with no increase in inhibitor titres.
Methods: Stage A: Four patients with a history of inhibitors were given single IV injections of PEGLip-FVIII (simoctocog alfa) at a dose of 22mg/kg PEGLip + 35 IU/kg FVIII and assessed for clotting activity at 0 hours (pre-injection) and at 20min, 1, 2, 4, 8, 24 hours, and daily thereafter up to 7 days using Rotational Thromboelastometry. Stage B: Patients received IV injections of PEGLip-FVIII for 6-weeks at a frequency determined by the investigator based on results obtained during Stage A. Inhibitor titres were monitored throughout.
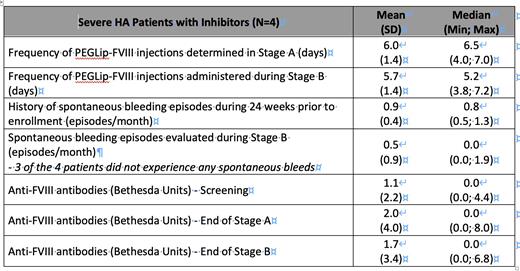
Results: Results are shown below.
Treatment with PEGLip-FVIII was highly tolerated with no clinically significant changes in inhibitor titres. No Adverse Drug Reactions were reported. The mean frequency of administration of PEGLip-FVIII was every 5.7±1.4 days. The mean number of bleeding episodes reported during Stage B was 0.5±0.9 per month (due to 1 patient) compared with 0.9±0.4 per month recorded during the 24 weeks prior to enrollment.
Conclusion: PEGLip-FVIII in inhibitor patients demonstrated efficacy in preventing spontaneous bleeds without increasing inhibitor titres, indicating a novel FVIII-based treatment for this cohort. Planned studies in a larger cohort may confirm our findings.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal